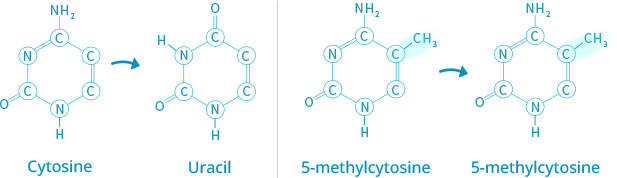
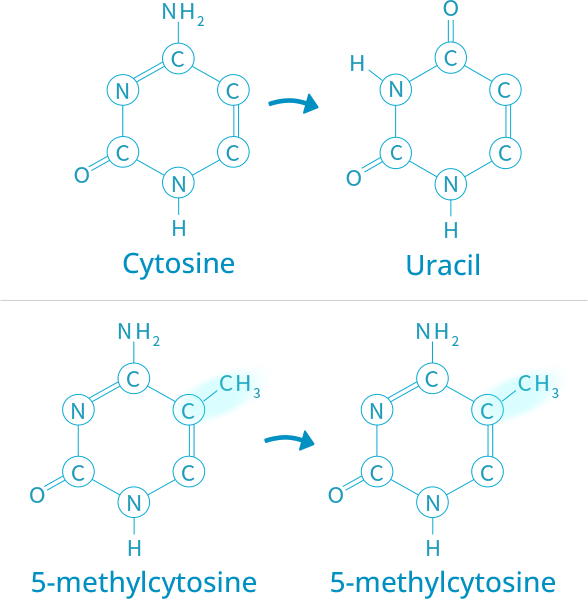
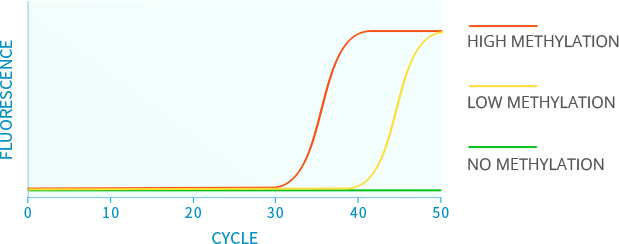
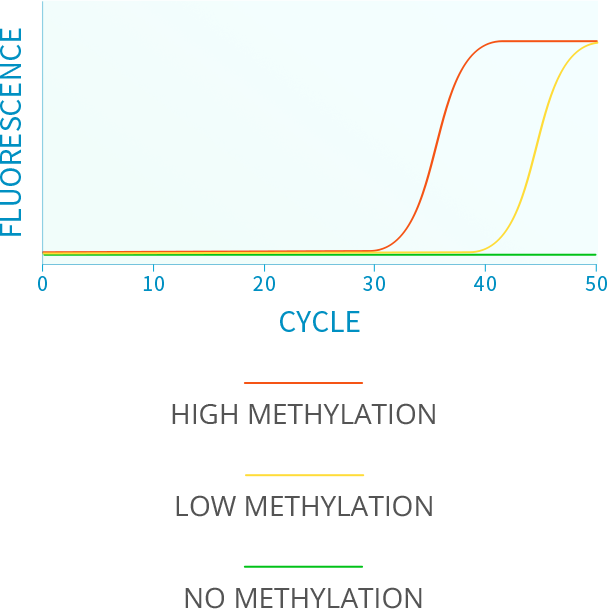
ABOUT ISTAT
diagnostic
products
products
healthcare
big data
big data
precision
medicine
medicine